AKTUELL-MELDUNGEN
Swiss Medtech sucht Leiter/in Geschäftsbereich Public Affairs, Mitglied der Geschäftsleitung
Adrian Hunn wird Swiss Medtech Direktor
1 X JA, 2 X NEIN
Nachhaltigkeit
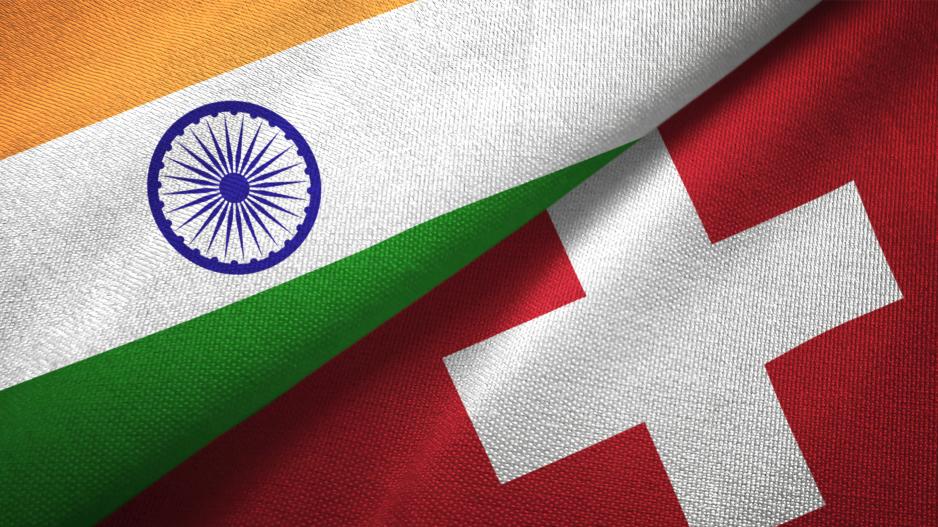
Swiss Medtech begrüsst Freihandelsabkommen mit Indien
Swiss Medtech Day 2024
Aktuellste Kennzahlen der Schweizer Medizintechnik-Industrie
VERANSTALTUNGSKALENDER
Swiss Medtech Webinar
29.04.
2024
Inverkehrbringen von Medizinprodukten in der Schweiz, auf Englisch
iCal
2024-04-29 02:00:00
2024-04-29 02:00:00
Inverkehrbringen von Medizinprodukten in der Schweiz
Inverkehrbringen von Medizinprodukten in der Schweiz https://www.swiss-medtech.ch/node/10
sitem-insel
Freiburgstrasse 3
0000 auf Englisch
Schweiz
Swiss Medtech
https://www.swiss-medtech.ch
Europe/Zurich
public
iCal
2024-04-30 02:00:00
2024-04-30 02:00:00
Inverkehrbringen von Medizinprodukten in der Schweiz
Inverkehrbringen von Medizinprodukten in der Schweiz https://www.swiss-medtech.ch/node/10
sitem-insel
Freiburgstrasse 3
0000 auf Deutsch
Schweiz
Swiss Medtech
https://www.swiss-medtech.ch
Europe/Zurich
public
iCal
2024-05-08 02:00:00
2024-05-08 02:00:00
Inverkehrbringen von Medizinprodukten in der Schweiz
Webinar zum Inverkehrbringen von Medizinprodukten in der Schweiz https://www.swiss-medtech.ch/node/10
sitem-insel
Freiburgstrasse 3
0000 auf Französisch
Schweiz
Swiss Medtech
https://www.swiss-medtech.ch
Europe/Zurich
public
iCal
2024-05-13 02:00:00
2024-05-13 02:00:00
La nuova fiscalità dei frontalieri: risvolti pratici e aspetti di cui tenere conto con un particolare riferimento al Medtech
La nuova fiscalità dei frontalieri: risvolti pratici e aspetti di cui tenere conto con un particolare riferimento al Medtech https://www.swiss-medtech.ch/node/10
Sala Aragonite
Via ai Boschetti 10
6928 Manno
Schweiz
Swiss Medtech
https://www.swiss-medtech.ch
Europe/Zurich
public
iCal
2024-05-23 02:00:00
2024-05-23 02:00:00
Swiss Medtech Mitgliederversammlung 2024
Die Swiss Medtech Mitgliederversammlung ist exklusiv für Mitarbeitende von Swiss Medtech Mitgliedsfirmen. Im Vorfeld der Swiss Medtech Mitgliederversammlung finden Versammlungen der Fachgruppen statt. https://www.swiss-medtech.ch/node/10
Swiss Medtech
Freiburgstrasse
3
3010 Bern
Schweiz
Swiss Medtech
https://www.swiss-medtech.ch
Europe/Zurich
public
iCal
2024-05-29 02:00:00
2024-05-29 02:00:00
Annual Biomedical Engineering Day
The 15th Biomedical Engineering Day of the University of Bern will take place on Wednesday, May 29, 2024. It is our pleasure to welcome you again at auditorium Ettore Rossi on Inselspital campus. https://www.swiss-medtech.ch/node/10
University of Bern
Freiburgstrasse
3010 Bern
Schweiz
Swiss Medtech
https://www.swiss-medtech.ch
Europe/Zurich
public